31 January, 2012
Commonly Abused Drugs | National Institute on Drug Abuse
Commonly Abused Drugs | National Institute on Drug Abuse
Sleep-Deprived Neurons Caught Nodding Off
A new study sheds light on how sleep deprivation might affect daily function. When researchers kept rats awake, they caught neurons in the thinking part of the animals’ brains taking catnaps. The finding gives insight into the roots of sleepiness.

It’s clear that people and other animals need sleep, but why is largely unknown. When you stay awake too long—even if you don’t feel sleepy—you can have attention lapses, show poor judgment and make frequent mistakes in cognitive tasks. Studies of brain activity in sleepy people suggest some similarities with that of people who are asleep. However, little has been known about the underlying neuronal activity at work.
A team led by Dr. Giulio Tononi of the University of Wisconsin-Madison set out to learn more about the sleepy brain. They kept rats awake for several hours by putting novel objects into their cages—colorful balls, boxes, tubes and nesting material from other rats. They used electroencephalography to track electrical activity at multiple sites in the brain’s cortex, which is important for thinking and cognition. The study was funded in part by NIH’s National Institute of Mental Health (NIMH) and National Institute of Neurological Disorders and Stroke (NINDS). The results appeared in the April 28, 2011, issue of Nature.
As the rats grew sleepy, subsets of cortex neurons switched off, seemingly at random, in various locations. The electrical profiles of these tired neurons showed “slow wave” activity, resembling neurons throughout the cortex during nonrapid-eye-movement (NREM) sleep, which makes up about 80% of all sleep. But the overall electrical activity in the rats’ brains confirmed that they were awake, as did their behavior.
To test if this neuronal “tiredness” affects performance, the researchers trained the rats during the sleep deprivation period to perform a task that involves reaching for a sugar pellet. A rat’s likelihood of success dropped by nearly 38% when neurons anywhere in the motor cortex turned off within a split second before a rat tried to reach for a sugar pellet. The overall number of such misses, as expected, increased significantly with prolonged wakefulness.
These results suggest that tired neurons might help to account for the impaired performance of sleep-deprived people. “Such tired neurons in an awake brain may be responsible for the attention lapses, poor judgment, mistake-proneness and irritability that we experience when we haven’t had enough sleep, yet don’t feel particularly sleepy,” explains Tononi. “Strikingly, in the sleep-deprived brain, subsets of neurons go offline in one cortex area but not in another—or even in one part of an area and not in another.”
Similar local neuron lapses, the researchers note, have been observed in epilepsy. That suggests this finding could hold significance for understanding not only sleepiness but also certain brain disorders.
Source:NIH
New Uses for Existing Medicines
In a novel approach, researchers used computers and genomic data to find new applications for existing FDA-approved drugs. The accomplishment represents a major step forward in drug discovery.

Drug approval takes many years of research, development and safety testing. When drugs that have already been approved are used for other purposes, it can avoid a great deal of time and investment. However, it’s difficult to figure out what other uses a given drug could serve. A team of researchers at Stanford University led by Dr. Atul J. Butte hypothesized that effective drugs might induce gene expression profiles that are opposite to the profiles caused by the condition they could treat. For example, if a disease increases the activity of certain genes, drugs that decrease the activity of those genes might be used to treat the disease.
To test this idea, the researchers drew on data from the NIH National Center for Biotechnology Information Gene Expression Omnibus, a publicly available database that contains the results of thousands of genomic studies submitted by researchers across the globe. This resource catalogs changes in gene activity under various conditions, such as in diseased tissues or in response to medications.
The research team created a computer program to compare the expression profiles of about 164 drugs and 100 diseases. The program searched through the thousands of possible drug-disease combinations to find drugs and diseases whose gene expression patterns essentially cancelled each other out. The work was supported by NIH’s National Institute of General Medical Sciences (NIGMS), National Cancer Institute (NCI) and National Library of Medicine (NLM). The results appeared in 2 articles on August 17, 2011, in Science Translational Medicine.
The approach pointed to potential drug-disease relationships for 53 of the 100 diseases examined. The program associated each of the 164 drugs with at least one disease. It predicted drug-disease pairs that are already in the market, validating the approach. For example, it matched prednisolone, a well known corticosteroid, with Crohn’s disease, for which it is already a standard therapy.
The researchers selected 2 candidate drug-disease pairs for further testing. They found that cimetidine, which is prescribed for heartburn, successfully inhibited the growth of human lung tumors both in the laboratory and when implanted in mouse. They also found that topiramate, an epilepsy drug, effectively decreased symptoms in rodents with inflammatory bowel disease.
In addition to identifying new potential drug-disease relationships, this approach may provide more basic insights. The program clustered drugs based on their mode of action. By studying unexpected members of these clusters, scientists could learn more about how certain diseases progress and how drugs work at the molecular level.
"This work is still in an early stage, but it is promising proofs of principle for a creative, fast and affordable approach to discovering new uses for drugs we already have in our therapeutic arsenal," says Dr. Rochelle M. Long, Director of the NIH Pharmacogenomics Research Network.
28 January, 2012
FDA approves Inlyta to treat patients with a type of advanced kidney cancer
Drug helps keep cancer from progressing
The U.S. Food and Drug Administration today approved Inlyta (axitinib) to treat patients with advanced kidney cancer (renal cell carcinoma) who have not responded to another drug for this type of cancer.
Renal cell carcinoma is a type of kidney cancer that starts in the lining of very small tubes in the kidney. Inlyta works by blocking certain proteins called kinases that play a role in tumor growth and cancer progression. Inlyta is a pill that patients take twice a day.
“This is the seventh drug that has been approved for the treatment of metastatic or advanced kidney cell cancer since 2005,” said Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “Collectively, this unprecedented level of drug development within this time period has significantly altered the treatment paradigm of metastatic kidney cancer, and offers patients multiple treatment options.”
Recently approved drugs for the treatment of kidney cancer include sorafenib (2005), sunitinib (2006), temsirolimus (2007), everolimus (2009), bevacizumab (2009) and pazopanib (2009).
The safety and effectiveness of Inlyta were evaluated in a single randomized, open-label, multi-center clinical study of 723 patients whose disease had progressed on or after treatment with one prior systemic therapy. The study was designed to measure progression-free survival, the time a patient lived without the cancer progressing. Results showed a median progression-free survival of 6.7 months compared to 4.7 months with a standard treatment (sorafenib).
The most common side effects observed in greater than 20 percent of patients in the clinical study were diarrhea, high blood pressure (hypertension), fatigue, decreased appetite, nausea, loss of voice (dysphonia), hand-foot syndrome (palmar-plantar erythrodysesthesia), weight loss, vomiting, weakness (asthenia) and constipation.
Patients with high blood pressure should have it well-controlled before taking Inlyta. Some patients who took Inlyta experienced bleeding problems, which in some cases were fatal. Patients with untreated brain tumors or gastrointestinal bleeding should not take Inlyta.
Inlyta is marketed by New York City-based Pfizer Inc.
For more information:
FDA: Office of Hematology and Oncology Products
Source: U.S. FDA
Buruli ulcer: from a difficult past to a hopeful future
This short advocacy film depicts the tragedy of Buruli ulcer, an infectious disease that destroys large areas of skin when detected and treated late. Against this tragic backdrop, the overarching message is one of hope and promise, thanks to the introduction of antibiotics. Using facts, figures, and moving testimonials, the film brings much good news.
For more information: http://www.who.int/buruli/en/#
Source: WHO
26 January, 2012
Everyone needs a flu vaccine!
Source: CDC
25 January, 2012
WHO: Saving mother's and children's lives
Produced in August 2011, this video highlights that every year, an estimated 360 000 women die in pregnancy and childbirth and around 8 million children die before their fifth birthday. Millions can be saved if the right health care is available.
WHO's department of maternal, newborn, child and adolescent health:
· generates and collect the latest evidence
· sets global standards
· helps to make treatments more affordable and effective
· provides guidance on delivering the best possible care
· designs training materials to give health workers the skills they need; and
· helps countries to get the right policies and programmes in place, and monitor progress
Help us to make sure that women and children everywhere enjoy their right to health.
(music in the video was kindly produced by Laurent Apfel/Lenz Music )
Source: WHO
India records one year without polio cases
One year polio free in India is a major achievement; the country was once the world’s epicentre of polio
2 JANUARY 2012 | ATLANTA/EVANSTON/GENEVA/NEW YORK/SEATTLE -India appears to have interrupted wild poliovirus transmission, completing one year without polio since its last case, in a 2-year-old girl in the state of West Bengal, on 13 January 2011.
India was once recognized as the world’s epicentre of polio. If all pending laboratory investigations return negative, in the coming weeks India will officially be deemed to have stopped indigenous transmission of wild poliovirus. The number of polio-endemic countries, those which have never stopped indigenous wild poliovirus transmission, will then be reduced to a historical low of three: Afghanistan, Nigeria and Pakistan.
However, there remains no room for complacency. India must maintain sensitive surveillance and high childhood immunity against wild poliovirus to guard against any importation of polio until eradication is achieved globally. In 2011, Afghanistan and Pakistan have both seen alarming increases in polio cases, and poliovirus from Pakistan re-infected China (which had been polio-free since 1999). In Africa, active polio transmission continues in Chad, the Democratic Republic of the Congo and Nigeria, with outbreaks in West and Central Africa in the past 12 months reminding the world that as long as polio exists anywhere, it remains a threat everywhere.
Global health leaders today paid tribute to the Government of India for its leadership and financial commitment to the polio eradication effort, and to the millions of vaccinators, community mobilizers, Rotarians, parents and caregivers who have supported polio eradication for more than a decade. The scale of the eradication effort in India is mind-boggling: each year, more than 170 million children under the age of 5 are vaccinated in two national immunization campaigns, with up to 70 million children in the highest-risk areas vaccinated multiple times in additional special campaigns; the whole effort requires nearly a billion doses of oral polio vaccine annually.
Hundreds of thousands of children will be saved
India’s achievement in stopping polio will save hundreds of thousands of children from lifelong paralysis or death each year. Poliovirus can travel easily to polio-free areas. Stopping polio in India will prevent a recurrence of the polio outbreaks – due to virus of Indian origin – seen in recent years in countries as diverse as Angola, Bangladesh, Nepal, Russia and Tajikistan.
An opportunity to end polio
"India’s success is arguably its greatest public health achievement and has provided a global opportunity to push for the end of polio," said WHO Director-General Margaret Chan. “The Global Polio Eradication Initiative is in full emergency mode and focused on using this momentum to close this crippling disease down. Stopping polio in India required creativity, perseverance and professionalism – many of the innovations in polio eradication were sparked by the challenges in India. The lessons from India must now be adapted and implemented through emergency actions to finish polio everywhere.”
The key to India’s remarkable progress in the fight against polio according to UNICEF Executive Director Anthony Lake, has been the strong leadership of the Government of India and state governments, which launched a comprehensive polio eradication programme that has enabled sustained high immunization coverage in states like Uttar Pradesh and Bihar with high rates of poverty, high population density and poor sanitation and infrastructure, conditions in which disease like polio can thrive.
Polio can be eradicated in challenging environments
“India’s achievement is proof positive that we can eradicate polio even in the most challenging environments – in fact, it is only by targeting these areas that we can defeat this evil disease,” Mr Lake said. “We have the ability to protect every last person, especially children, from this entirely preventable disease – and because we can, we must finish the job of eradicating polio globally, once and for all."
Rotary International first launched the global polio eradication effort in 1985, and President Kalyan Banerjee said that with the intensity of transmission in India, many experts had predicted it would be the last country in the world to achieve eradication. “India is undoubtedly the biggest domino to fall in the polio eradication effort,” Mr Banerjee said. “India’s success is a great credit to the Indian government and to Indian Rotary members – as well as those from around the world – who have worked with local leaders to conduct these immunization efforts to reach every child with the polio vaccine."
India must continue to protect its children from polio
Like all countries that have stopped indigenous wild poliovirus transmission, India must continue to protect its children through supplementary immunization activities and improved routine immunization coverage rates or risk a potentially horrific re-importation event, said the Director of the U.S. Centers for Disease Control and Prevention, Dr. Thomas Frieden. “Polio’s history contains many cautionary tales,” Dr. Frieden added. “Polio anywhere in the world is a risk everywhere in the world, and to protect itself from a setback, India is appropriately planning to continue meticulous monitoring and intensive childhood vaccination against polio.”
Ensuring no child suffers polio
“Polio can be stopped when countries combine the right elements – political will, quality immunization campaigns, and an entire nation’s determination” said Bill Gates, co-chair of the Bill & Melinda Gates Foundation. “World leaders must continue to raise the funds needed to run the global campaign and help to ensure that no child suffers from this crippling disease ever again.”
With India’s achievement, the global polio eradication effort now focuses on improving the implementation of emergency operations plans in Chad, Nigeria and Pakistan. Success depends on local ownership and accountability at all levels of government and international partners.
Unite in the fight against NCDs
Thirty-six million people die each year from noncommunicable diseases (NCDs) like heart disease and stroke, diabetes, cancer and chronic lung disease. On 19-20 September 2011, global leaders met at the United Nations in New York to turn the tide on NCDs.
Source: WHO
Information by Drug Class Influenza (Flu) Antiviral Drugs and Related Information
There are a number of drugs approved by FDA for the treatment and prevention of influenza. Vaccination is the primary means of preventing and controlling influenza.
Information by Drug Class Influenza (Flu) Antiviral Drugs and Related Information
22 January, 2012
21 January, 2012
THE VERITAS HEALTHCARE SOLUTIONS LLC: Site management Organization Stepping from USA to India
The Veritas Healthcare Solutions is a full line Clinical Research Consulting and Site Management Organization that specializes in providing solutions to the twin challenges of speed and cost in clinical drug development. We provide cost effective solutions and full research support from Phase I to Phase IV clinical trials to the global pharmaceutical, biotechnological (or biopharmaceutical) and medical device companies.
You might be aware of the fact that Clinical Research is a Multi Billion Dollar industry and growing at a phenomenal rate. There are ample opportunities for qualified professionals who are adequately trained in Clinical research to have a fulfilling and financially rewarding career in this industry.
India is perceived to be the future global Hub for the Clinical Research Industry and a preferred destinations for Employers seeking professionals in the field of Healthcare and Clinical Research.
VERITAS HealthCare LLC has taken an initiative with the objective of providing quality training to healthcare /pharma and other life science graduates in the field of Clinical research to meet the demands of the Industry.
The training is designed as an online interactive program with 8 sessions spread over two months on weekends (2-3Hrs/week with an optional three month internship). Further details of program is attached with this mail.
It shall also remain the endeavour of the company to guarantee Job opportunities to the qualified candidates.
For further details please contact...
Cell: +91 8129322885, 9895322885
Phone: 0484 4032885, 4042885.
Email: kochi@theveritas.co.in , Web: www.theveritashealthcare.com
Kochi Office: Mayur Business Centre, Chittoor Road, Pulleppady Jn, Kochi: 682035, India T: (0484) 403 2885
US Office: The Veritas Healthcare Solutions, 469 7th Ave, 3rd Fl(between 36th & 35th St), New York, NY 10018, USA
Indian HO: SCO 20 (2nd) FL Phase 3B2, Mohali,India, 160059, India T: (0172)-4662885 , 6532885 , 5044885
17 January, 2012
12 January, 2012
Survival Tactics of a Common Gut Microbe
In a recent mouse study, scientists discovered how a common gut bacterium sends a “do not attack” signal to the immune system. The finding helps explain how our bodies distinguish between harmful microbes and those essential for health.

Image: Microbes colonizing the surface of the mouse colon. Yellow cells areEscherichia coli; red cells areBacteroides fragilis. Intestinal tissues are labeled in green with blue nuclei.Credit: S. Melanie Lee/Caltech.
Trillions of microbes thrive in our gut. They can help us digest our food, prevent infections, and may even affect our risk of developing autoimmune diseases. These helpful microbes remain relatively undisturbed by the human immune system. But small numbers of disease-causing microbes, like salmonella, elicit attacks from these same immune defenses. How the immune system knows which to attack and which to ignore—especially when friend and foe can look very similar—has been a long-standing mystery.
To investigate, a research team led by Dr. Sarkis K. Mazmanian of the California Institute of Technology studied a common friendly, or commensal, gut bacterium, Bacteroides fragilis, in the mouse gut. The study was funded in part by NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Allergy and Infectious Diseases (NIAID). The report appeared online on April 21, 2011, in Science.
To find out how B. fragilis avoids attack, the team studied germ-free mice whose guts were colonized with B. fragilis. The colonized mice did not show an increase in T helper 17 (Th17) cells—immune cells important for eliminating harmful pathogens.
In a previous study, Mazmanian had shown that the beneficial effect of B. fragilis requires a molecule named Polysaccharide A (PSA). When the researchers removed PSA from B. fragilis, they found that mouse Th17 cells responded.
Next, to determine how B. fragilis suppress Th17 cells, the researchers inactivated components of the animals’ immune response. Signaling through Toll-like receptors has been shown to both activate and restrain immune responses, and PSA is known to act through the mouse Toll-like receptor 2 (TLR2). When the researchers inactivated mouse TLR2, the number of Th17 cells increased, and the cells prevented intestinal colonization of B. fragilis. Similarly, cells called Treg cells normally suppress Th17 responses to prevent the immune system from attacking its own tissues. When the researchers inactivated mouse Treg cells, they also saw an increase in Th17 cells and a reduction in B. fragilis intestinal colonization.
Researchers had previously thought that commensal gut bacteria avoided immune detection by living far away from the surface of the intestine. Using a 3D microscopy technique, Mazmanian’s team found that B. fragilisoccupies a unique niche within the mucosal lining of the mouse’s colon, in close contact with the immune system.
Taken together, the results suggest that B. fragilis evades the gut’s immune response by coercing the immune system into activating Treg cells. It does this by producing PSA, which is detected in the mouse intestine by TLR2.
“These bacteria live inside us for our entire lives, and they’ve evolved to look and act like us, as part of us,” says Mazmanian. “As far as our immune system is concerned, the molecules made by gut bacteria should be tolerated similarly to our own molecules. Except in this case, the bacteria 'teach' us to tolerate them, for both our benefit and theirs.”
—by Amy Alabaster
Source: NIH
Breaking Bad Habits
Why It’s So Hard to Change

If you know something’s bad for you, why can’t you just stop? About 70% of smokers say they would like to quit. Drug and alcohol abusers struggle to give up addictions that hurt their bodies and tear apart families and friendships. And many of us have unhealthy excess weight that we could lose if only we would eat right and exercise more. So why don’t we do it?
NIH-funded scientists have been searching for answers. They’ve studied what happens in our brains as habits form. They’ve found clues to why bad habits, once established, are so difficult to kick. And they’re developing strategies to help us make the changes we’d like to make.
“Habits play an important role in our health,” says Dr. Nora Volkow, director of NIH’s National Institute on Drug Abuse. “Understanding the biology of how we develop routines that may be harmful to us, and how to break those routines and embrace new ones, could help us change our lifestyles and adopt healthier behaviors.”
Habits can arise through repetition. They are a normal part of life, and are often helpful. “We wake up every morning, shower, comb our hair or brush our teeth without being aware of it,” Volkow says. We can drive along familiar routes on mental auto-pilot without really thinking about the directions. “When behaviors become automatic, it gives us an advantage, because the brain does not have to use conscious thought to perform the activity,” Volkow says. This frees up our brains to focus on different things.
Habits can also develop when good or enjoyable events trigger the brain’s “reward” centers. This can set up potentially harmful routines, such as overeating, smoking, drug or alcohol abuse, gambling and even compulsive use of computers and social media.
“The general machinery by which we build both kinds of habits are the same, whether it’s a habit for overeating or a habit for getting to work without really thinking about the details,” says Dr. Russell Poldrack, a neurobiologist at the University of Texas at Austin. Both types of habits are based on the same types of brain mechanisms.
“But there’s one important difference,” Poldrack says. And this difference makes the pleasure-based habits so much harder to break. Enjoyable behaviors can prompt your brain to release a chemical called dopamine. “If you do something over and over, and dopamine is there when you’re doing it, that strengthens the habit even more. When you’re not doing those things, dopamine creates the craving to do it again,” Poldrack says. “This explains why some people crave drugs, even if the drug no longer makes them feel particularly good once they take it.”
In a sense, then, parts of our brains are working against us when we try to overcome bad habits. “These routines can become hardwired in our brains,” Volkow says. And the brain’s reward centers keep us craving the things we’re trying so hard to resist.
The good news is, humans are not simply creatures of habit. We have many more brain regions to help us do what’s best for our health.
“Humans are much better than any other animal at changing and orienting our behavior toward long-term goals, or long-term benefits,” says Dr. Roy Baumeister, a psychologist at Florida State University. His studies on decision-making and willpower have led him to conclude that “self-control is like a muscle. Once you’ve exerted some self-control, like a muscle it gets tired.”
After successfully resisting a temptation, Baumeister’s research shows, willpower can be temporarily drained, which can make it harder to stand firm the next time around. In recent years, though, he’s found evidence that regularly practicing different types of self-control—such as sitting up straight or keeping a food diary—can strengthen your resolve.
“We’ve found that you can improve your self-control by doing exercises over time,” Baumeister says. “Any regular act of self-control will gradually exercise your ‘muscle’ and make you stronger.”
Volkow notes that there’s no single effective way to break bad habits. “It’s not one size fits all,” she says.
One approach is to focus on becoming more aware of your unhealthy habits. Then develop strategies to counteract them. For example, habits can be linked in our minds to certain places and activities. You could develop a plan, say, to avoid walking down the hall where there’s a candy machine. Resolve to avoid going places where you’ve usually smoked. Stay away from friends and situations linked to problem drinking or drug use.
Another helpful technique is to visualize yourself in a tempting situation. “Mentally practice the good behavior over the bad,” Poldrack says. “If you’ll be at a party and want to eat vegetables instead of fattening foods, then mentally visualize yourself doing that. It’s not guaranteed to work, but it certainly can help.”
One way to kick bad habits is to actively replace unhealthy routines with new, healthy ones. Some people find they can replace a bad habit, even drug addiction, with another behavior, like exercising. “It doesn’t work for everyone,” Volkow says. “But certain groups of patients who have a history of serious addictions can engage in certain behaviors that are ritualistic and in a way compulsive—such as marathon running—and it helps them stay away from drugs. These alternative behaviors can counteract the urges to repeat a behavior to take a drug.”
Another thing that makes habits especially hard to break is that replacing a first-learned habit with a new one doesn’t erase the original behavior. Rather, both remain in your brain. But you can take steps to strengthen the new one and suppress the original one. In ongoing research, Poldrack and his colleagues are using brain imaging to study the differences between first-learned and later-learned behaviors. “We’d like to find a way to train people to improve their ability to maintain these behavioral changes,” Poldrack says.
Some NIH-funded research is exploring whether certain medications can help to disrupt hard-wired automatic behaviors in the brain and make it easier to form new memories and behaviors. Other scientific teams are searching for genes that might allow some people to easily form and others to readily suppress habits.
Bad habits may be hard to change, but it can be done. Enlist the help of friends, co-workers and family for some extra support.
Break Bad Habits
- Avoid tempting situations. If you always stop for a donut on your way to work, try a different route. Keep fatty foods, cigarettes, alcohol and other tempting items out of your home.
- Replace unhealthy behaviors with healthy ones. Try exercise, a favorite hobby or spending time with family.
- Prepare mentally. If you can’t avoid a tempting situation, prepare yourself in advance. Think about how you want to handle it and mentally practice what you plan.
- Enlist support. Ask friends, family and co-workers to support your efforts to change.
- Reward yourself for small steps.Give yourself a healthy treat when you’ve achieved a small goal or milestone.
Source: NIH
11 January, 2012
Saliva Testing Catches CMV Infection in Newborns
A saliva sample from a newborn can be used to quickly and effectively detect cytomegalovirus (CMV) infection, a major cause of hearing loss in children. Better CMV screening could help doctors know which patients to monitor for symptoms so they can be treated as quickly as possible.

Image by Anthea Sieveking, all rights reserved by Wellcome Images.
CMV is the most common infection passed from a mother to her unborn child. Between 20,000 and 30,000 infants are infected with the virus upon birth, and 10% to 15% of them are at risk for developing hearing loss. Monitoring infected children for signs of hearing loss as they grow is the best way to ensure they get early treatment. But infected babies often show no symptoms, so screening for CMV at birth is critical.
CMV infection is currently detected using the “rapid culture” test. However, this method isn’t easily automated to allow for widespread screening. A team at the University of Alabama at Birmingham, led by Dr. Suresh Boppana and Dr. Karen Fowler, set out to develop a fast, accurate test to identify CMV-infected newborns. They used a technique called polymerase chain reaction (PCR), which amplifies virus DNA and could be used to screen large numbers of infants for CMV. The study was funded by NIH’s National Institute on Deafness and Other Communication Disorders (NIDCD).
The researchers had previously shown that testing heel-stick blood by PCR accurately predicted CMV infection only 30% to 40% of the time, but 95% accuracy is required for a screening test. In an attempt to improve this percentage, the team tested saliva instead. They collected saliva via mouth swab from nearly 35,000 newborn infants at 7 hospitals and sent them to the laboratory for testing. In the first phase of the study, the samples were stored in liquid, and in the second phase, they were air-dried. The findings were published in the June 2, 2011, issue of the New England Journal of Medicine.
Among liquid saliva samples, the PCR and rapid culture tests both identified CMV in the same 85 infants, suggesting that the PCR test is 100% accurate compared to the current standard. Among dried saliva samples, rapid culture identified 76 infected infants. PCR identified 74 of these same infants, dropping its accuracy rate slightly to 97.4%.
Sixteen additional liquid saliva samples tested positive with the PCR test but not the rapid culture one. Follow-up analysis revealed that 3 of these were indeed positive, leading the researchers to conclude that PCR may be superior to rapid culture for CMV screening. For the remaining 13 samples, CMV may have been present in the mother’s breast milk or secretions in the birth canal that showed up in the baby’s mouth swab. For this reason, the researchers suggest that all positive results be confirmed with follow-up testing within the first 3 weeks of life.
“We now know that we have a test with saliva that works,” Boppana says. “The challenge is, unlike the dried blood spot, which is already used for newborn screening in hospitals across the country, we don’t have a system in place for the collection of saliva. But we’ve shown that if you wanted to test a lot of babies for congenital CMV infection, it can be done.”
The CMV-infected infants from this study are now enrolled in a follow-up program to see how strongly CMV infection at birth contributes to hearing loss in early childhood.
Source: NIH
Insulin Nasal Spray Shows Promise for Alzheimer’s Disease
A small clinical trial has found that daily doses of an insulin nasal spray can slow memory loss and preserve thinking skills in people with mild to moderate Alzheimer’s disease. A larger study is needed, though, to confirm the effectiveness of this therapy.

Alzheimer’s disease is a complex, irreversible brain disease that slowly destroys memory and thinking skills. Symptoms usually begin to appear after age 60. Although the cause of Alzheimer’s disease is unknown, researchers suspect that genes, metabolism, environment and lifestyle factors all play a role.
Over the past decade, some scientists have found evidence that abnormal functioning of the hormone insulin might contribute to memory loss and Alzheimer’s disease. Insulin helps to convert blood sugar, or glucose, into energy, and it’s known to be important to normal brain functioning.
Dr. Suzanne Craft of the Veterans Affairs Puget Sound Health Care System and her colleagues have been working to see if restoring normal insulin function to the brain might provide cognitive benefits and slow the progression of Alzheimer’s disease. Their research is supported in part by NIH’s National Institute on Aging (NIA) and the U.S. Department of Veterans Affairs.
Their new study examined the effects of an insulin nasal spray on 104 people with mild to moderate Alzheimer’s disease or mild cognitive impairment, a condition marked by memory problems that may eventually progress to Alzheimer’s disease. The nasal spray was designed to deliver insulin quickly and directly to the brain without causing harmful changes to blood insulin or glucose levels throughout the body.
Participants were randomly assigned to receive daily doses of either 20 IU (international units) of insulin, 40 IU of insulin or a saline placebo administered through a nasal drug delivery device. Memory, cognition and functional ability were measured before and after the 4-month treatment period.
As reported in the September 12, 2011, online edition of the Archives of Neurology, the researchers found that both doses of insulin preserved general functional abilities, or activities of daily living, as assessed by caregivers. In addition, treatment with 20 IU of intranasal insulin led to improved memory. However, no memory improvement was seen in the placebo group or in those receiving 40 IU of insulin, which suggests that this dose may exceed the optimal dose for memory. No harmful side effects were observed in the insulin-treated groups.
“The results are very promising. Not only did we see improved memory in one group, but there also seemed to be improved general functioning in both groups receiving insulin,” says Dr. Laurie Ryan, who oversees Alzheimer’s disease clinical trials supported by NIA. “Still, it’s important to remember that this research is still in its early stages. The findings highlight the need for a larger study, with more patients and a longer duration of treatment, to take a definitive look at whether or not this treatment will be effective.”
Source: NIH
DNA Primer Boosts Antibodies Against Avian Flu
Production of avian flu-fighting antibodies rose significantly when healthy adults were given a DNA “primer” vaccine 6 months before receiving an avian flu vaccine. This type of regimen might improve protection against avian flu and other strains of influenza.

Image: A 3D graphical representation of an influenza virus. Image by Dan Higgins, courtesy of CDC.
The avian flu virus, known as H5N1, poses a major threat to public health. The virus originated in birds, but some strains are now able to infect humans. Most people have no pre-existing immunity to H5N1, as they do for the annually recurring seasonal flu.
An avian flu vaccine made of a whole, inactivated H5N1 virus can trigger the immune system to produce modest levels of protective antibodies. Scientists have been seeking ways to elicit more effective protection against H5N1 as well as other influenza viruses.
Last year, animal studies by NIH scientists showed that the antibody response to seasonal flu vaccines could be enhanced by first giving a primer vaccine. The primer included a piece of DNA that encodes a key viral-surface protein called hemagglutinin (HA). Seasonal flu vaccines typically spur production of antibodies that latch onto the globular head region of HA, an area that constantly changes. In animals, adding a DNA primer to the vaccine regimen elicited broadly neutralizing antibodies against the HA stem, which remains relatively constant across many strains of influenza.
To see if the DNA primer method might work in humans, Dr. Gary Nabel of NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and his colleagues developed a DNA vaccine featuring the HA gene from H5N1. The scientists conducted 2 clinical trials to assess the safety and effects of the new regimen. Their research is described in the October 4, 2011, online edition of Lancet Infectious Diseases.
The 2 trials enrolled a total of 81 adults. Some were randomly assigned to receive a DNA primer followed by a booster vaccine made of whole, inactivated H5N1. The booster was given either 1 month or 6 months after the DNA primer. For comparison, other volunteers received 2 doses of the inactivated H5N1 vaccine given at either 1- or 6-month intervals.
The researchers found that the DNA primer was safe, and the 6-month interval between primer and H5N1 booster triggered the most protective antibody response. Of the 26 volunteers from both trials who received the DNA primer followed by the booster 6 months later, 21 produced antibodies at levels predicted to protect them from H5N1 influenza. The antibody levels in that group were more than 4 times higher than those in people who received 2 doses of the inactivated H5N1 vaccine. In some volunteers, the prime-boost vaccine regimen also spurred production of broadly neutralizing antibodies aimed at the HA stem.
The scientists are now working to improve their DNA vaccines to more readily elicit antibodies directed at the stem region of the HA protein. NIH researchers are also planning a larger trial of a prime-boost vaccine for seasonal influenza.
“The results of these studies demonstrate an important proof of concept, in that it is possible to elicit broadly neutralizing influenza antibodies in humans through vaccination,” says NIAID Director Dr. Anthony S. Fauci. “These findings mark an early but significant milestone on the pathway to a universal influenza vaccine that provides protection against multiple virus strains.”
Source: NIH
7 Must Know Safety Tips for Hospital Patients
We’ve all heard of medical horror stories that end in disaster, but did you know that there are things you can do as a hospital patient to lower your chances of becoming one of them? Far from earning a medical degree yourself, there are many simple things any patient can do to make their hospital stay as effective as possible. To help, we have gathered seven tips that anyone going into a hospital should know.
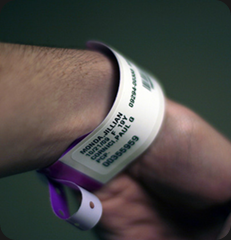 List allergies – One of the most common hospital mistakes, patients who are allergic to certain kinds of medication will accidentally be given it. Make sure you alert hospitals to all allergies when checking in. You can even request colored allergy bracelets to wear at all times.
List allergies – One of the most common hospital mistakes, patients who are allergic to certain kinds of medication will accidentally be given it. Make sure you alert hospitals to all allergies when checking in. You can even request colored allergy bracelets to wear at all times. - List current medications – If you are one of the millions of Americans who take prescription medication, don’t be shy when checking into a hospital. Some medications can be harmful when taken with others, and it is important for a hospital staff to know. It is also a good idea for you to know which medications you should not be taking.
- Use the bracelet – That little bracelet you get on checking into a hospital is there for a reason. With the use of bar code technology, nurses and doctors can easily see what you’ve been taking and are about to take, so make sure they scan each time.
- Talk to your doctors – Make sure your hospital doctor knows why you are there at all times. Miscommunication can lead to medical errors in a serious way. Also be sure that you understand what tests, diagnosis, or treatments you receive before continuing any plan.
- Talk to surgeons – If you are in the hospital to have a surgery, be sure to speak to the surgeon doing the procedure. This way, he or she can know exactly what is going to be done, as well as the face of who they are doing it to.
- Hygiene – Because infections are the number one killers in hospitals, make sure you, your medical staff, and your visitors are washing their hands, covering sneezes, etc. before touching you. It’s also a good idea to stay away from doctors with ties.
Source: http://www.pharmacydegrees.net
Paige Dagmar is a student and also writes for <a href=”>Pharmacy Degrees</a> which helps students find the right pharmacy degree
08 January, 2012
Beware of Fraudulent Weight-Loss ‘Dietary Supplements’
Magic diet pill!
Melt your fat away!
Diet and exercise not required!
Messages like these on weight-loss products taunt consumers looking for a quick and easy way to shed pounds.
But these products don’t live up to their claims. Even worse, they can cause serious harm, say federal regulators, who have found dozens of products being touted as dietary supplements but that actually contain hidden prescription drugs or compounds that have not been adequately studied in humans.
“These products are not legal dietary supplements,” says Michael Levy, director of the Food and Drug Administration’s (FDA’s) Division of New Drugs and Labeling Compliance. “They are actually very powerful drugs masquerading as ‘all-natural’ or ‘herbal’ supplements, and they carry significant risks to unsuspecting consumers."
“We have seen deaths associated with these weight-loss products,” adds Levy. “Make no mistake—they can kill you.”
Source: U.S FDA
FDA Warns About Stem Cell Claims

Researchers hope that stem cells will one day be effective in the treatment of many medical conditions and diseases.
Stem cell therapies offer the potential to treat diseases or conditions for which few treatments exist.
Stem cells, sometimes called the body’s “master cells,” are the precursor cells that develop into blood, brain, bones and all of your organs. Their promise in medical treatments is that they have the potential to repair, restore, replace and regenerate cells that could then be used to treat many medical conditions and diseases.
But the Food and Drug Administration (FDA) is concerned that the hope that patients have for cures not yet available may leave them vulnerable to unscrupulous providers of stem cell treatments that are illegal and potentially harmful.
FDA cautions consumers to make sure that any stem cell treatment they are considering has been approved by FDA or is being studied under a clinical investigation that has been submitted to and allowed to proceed by FDA.
FDA has approved only one stem cell product, Hemacord, a cord blood-derived product manufactured by the New York Blood Center and used for specified indications in patients with disorders affecting the body’s blood-forming system.
Regulation of Stem Cells
FDA regulates stem cells in the U.S. to ensure that they are safe and effective for their intended use.
“Stem cells can come from many different sources and under the right conditions can give rise to many different cell types,” says Stephanie Simek, Ph.D., deputy director of FDA’s Office of Cellular, Tissue and Gene Therapies.
Stem cells that come from bone marrow or blood are routinely used in transplant procedures to treat patients with cancer and other disorders of the blood and immune system.
Umbilical cord blood is collected from a placenta with the birth mother’s consent. Cord blood cells are then isolated, processed, and frozen and stored in a cord blood bank for future use. Cord blood is regulated by FDA and cord blood banks must follow regulatory requirements.
But there are many other stem cell products, including other cord blood-derived products, that have been reviewed by FDA for use in investigational studies, says Simek. Investigational products undergo a thorough review process as the sponsor prepares to study the safety and effectiveness of the product in adequate and well-controlled human studies (clinical trials).
As part of this review, the sponsor must show how the product will be manufactured so that FDA can make certain that appropriate steps are being taken to help assure the product’s safety, purity and potency. FDA also requires that there be sufficient data generated from animal studies to aid in evaluating any potential risks associated with the use of these products.
Consumers need to be aware that at present--other than cord blood for certain specified indications--there are no approved stem cell products.
Advice for Consumers
- If you are considering stem cell treatment in the U.S., ask your physician if the necessary FDA approval has been obtained or if you will be part of an FDA-regulated clinical study. This also applies if the stem cells are your own. Even if the cells are yours, there are safety risks, including risks introduced when the cells are manipulated after removal.
There is a potential safety risk when you put cells in an area where they are not performing the same biological function as they were when in their original location in the body,” says Simek. Cells in a different environment may multiply, form tumors, or may leave the site you put them in and migrate somewhere else. - If you are considering having stem cell treatment in another country, learn all you can about regulations covering the products in that country. Exercise caution before undergoing treatment with a stem cell-based product in a country that—unlike the U.S.—may not require clinical studies designed to demonstrate that the product is safe and effective. FDA does not regulate stem cell treatments used in solely in countries other than the United States and typically has little information about foreign establishments or their stem cell products.
Thwarting a Stem Cell Scheme
In December, 2011, three men were arrested in the United States and charged with 15 counts of criminal activity related to manufacturing, selling and using stem cells without FDA sanction or approval.
According to the criminal indictment, one of the accused, a licensed midwife who operated a maternity care clinic in Texas, obtained umbilical cord blood from birth mothers, telling them it was for “research” purposes. Instead, the midwife sold the cord blood to a laboratory in Arizona which, in turn, sent the blood to a paid consultant at a university in South Carolina. The owner of the laboratory in Arizona was convicted in August 2011 of unlawfully introducing stem cells into interstate commerce. She faces up to 3 years in prison and a fine of up to $10,000.
The consultant, an assistant professor, used university facilities to manufacture stem cell products. He then sent the products back to the lab, which sold them to a man representing himself as a physician licensed in the U.S. The man then traveled to Mexico to perform unapproved stem cell procedures on people suffering from cancer, multiple sclerosis and other autoimmune diseases.
The three defendants allegedly received more than $1.5 million from patients seeking treatment for incurable diseases.
“Scammers like these offer false hope to people with incurable diseases in order to line their own pockets with money,” says Special Agent in Charge Patrick J. Holland of FDA’s Office of Criminal Investigations (OCI), Kansas City Field Office. “FDA will continue to aggressively pursue perpetrators who expose the American public to the dangers of unapproved stem cells and ensure that they are punished to the full extent of the law.”
FDA’s OCI worked the case with the Federal Bureau of Investigations and the Internal Revenue Service’s Criminal Investigations Division.
Source: U.S FDA
04 January, 2012
The Straight Poop on Kids and Diarrhea

Diarrhea is never a good thing, but for an infant or toddler, it can be very bad—even life-threatening.
If the bout of loose, watery stools lasts more than a day, young children run the risk of dehydration—the loss of essential fluids that contain salts and other minerals needed for the body to function properly. Frequent, loose stools along with repeated vomiting may also be cause for concern.
Many over-the-counter (OTC) products can provide relief to adults and older children. But giving an infant or toddler one of these anti-diarrheal products can be harmful, says Benjamin Ortiz, M.D., a pediatrician in the Food and Drug Administration’s Office of Pediatric Therapeutics.
“The most important aspect of treating diarrhea is knowing the signs of dehydration and taking steps to rehydrate the child,” says Ortiz.
Signs of Dehydration
The early signs of dehydration in infants and young children include
- faster heartbeat than normal
- dry lips, mouth and tongue
- no tears when crying
- no wet diapers for 3 hours or more
Later in the process of dehydration, the child may have
- sunken eyes, cheeks, or soft spot on the top of the head
- sleepiness and irritability
Severe dehydration can cause seizures, coma, organ failure, and, in rare circumstances, death.
Rehydration
“Mild diarrhea is a discomfort, but not dangerous if the child can continue to drink fluids and eat a regular diet,” says Ortiz. Infants should continue to be given breast milk or their usual formula. If diarrhea persists or is frequent, a change in diet and treatment with oral rehydration solutions may be necessary.
Oral rehydration solutions, also called electrolyte solutions, help to replace the water and salts lost during diarrhea, and they may be easier to digest than the child’s regular diet. They often come in liquid or popsicle forms, and in different flavors. Some common rehydration products are Pedialyte, Naturalyte, Enfalyte, and CeraLyte. You can find these OTC products in drugstores and some retail stores.
“At first onset, try to encourage the child to drink as much of an oral rehydration solution or a regular diet as possible, even a few ounces every 15 to 30 minutes is good,” says Ortiz. “But if your child is persistently vomiting and can’t hold anything down, call your pediatrician or go to the ER.” Vomiting will speed up dehydration.
Avoid using home remedies for diarrhea like boiled milk or rice water. “Sports drinks are also not recommended for young children,” says Ortiz. “They tend to have extra sugar in them to help athletes during vigorous physical activity, but they are not an appropriate replacement fluid. Foods and fluids with higher sugar content, such as juice, cookies, cakes and sodas, can contribute to the diarrhea by pulling more fluid into the intestines, causing the excess fluid and sugar to come out the other end quickly.”
Ortiz also notes, “The beauty of oral rehydration products is that they have just enough sugar to allow for the absorption of sodium, potassium, and water without causing more diarrhea.”
Parents should discuss any concerns about worsening symptoms or the risk of dehydration with their child's pediatrician, adds Ortiz.
Over-the-Counter Medicines
Don't use OTC anti-diarrheal medicines in young children unless recommended by your child’s doctor, says Ortiz. Products to relieve diarrhea, such as Pepto-Bismol and Kaopectate, contain bismuth, magnesium, or aluminum, which can be harmful to infants and toddlers because they can quickly accumulate in young children’s bodies.
These products may be used in older children, but ask your pediatrician or read the packaging first for directions. Pepto-Bismol’s packaging directs you to ask a doctor before giving to children under 12. And Imodium’s packaging says to ask your doctor before giving to children under 6.
When to See the Doctor
According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), children with any of the following symptoms should see a doctor:
- signs of dehydration
- diarrhea for more than 24 hours
- fever of 102 degrees or higher
- stools that are black and tarry
- stools containing blood or pus
Blood rarely appears in the stools of infants and children, says Ortiz. “What looks like blood in the stool may be irritation of the anorectal area, causing bleeding of the skin. It appears as bright red blood that sits on top of the stool.” Your pediatrician can suggest a cream to provide relief if that is occurring.
Causes of Diarrhea
Acute diarrhea (comes on rapidly, is severe, but short in duration) is usually caused by a bacterial, viral, or parasitic infection, according to NIDDK.
Rotavirus, a virus that inflames the stomach and intestines, was the leading cause of acute diarrhea in U.S. children before a vaccine was introduced in 2006. FDA has licensed two rotavirus vaccines to prevent this infection. The liquid vaccine is given by mouth to infants between the ages of 6 weeks and 32 weeks in a series of two or three doses, depending on which of the two vaccines is used.
A vaccine, like any medicine, could possibly cause severe allergic reactions or other serious problems. Some studies have shown a small increase in cases of intussusception—a rare but serious bowel blockage—in infants after getting the rotavirus vaccine. But the risk of serious harm from the vaccine is very small and the benefit outweighs this risk. Rotavirus vaccination has reduced the number of babies needing emergency care or hospitalization for rotavirus disease by about 85 percent, according to the Centers for Disease Control and Prevention.
Chronic (ongoing or recurring) diarrhea or diarrhea that lasts more than one week may be related to other problems. “If you believe that your child has chronic diarrhea, see a pediatrician,” says Ortiz.
Source: U.S. FDA
FDA Approves First Scorpion Sting Antidote

Photo courtesy of J. Zirato, University of Arizona BioCommunications
“Once stung, twice shy” are words to live by in the Southwestern United States, where about 11,000 people a year are stung by scorpions in Arizona alone.
Though rarely life threatening, scorpion stings can be extremely painful, causing numbness and burning at the wound site. And there’s been little a victim could do to ease the pain.
Until now.
The Food and Drug Administration has just approved the first treatment specifically for the sting of the Centruroides scorpion, the most common type in the United States.
The new biologic treatment—called Anascorp—was given a priority review because adequate treatment did not exist in the United States, says Karen Midthun, M.D., director of the FDA’s Center for Biologics Evaluation and Research.
“This product provides a new treatment for children and adults and is designed specifically for scorpion stings,” Midthun says. “Scorpion stings can be life-threatening, especially in infants and children.”
Severe stings can cause loss of muscle control and difficulty breathing, requiring heavy sedation and intensive care in a hospital. Most often, it’s small children who experience severe reactions, but adults can be affected, too, says Keith Boesen, managing director of the Arizona Poison and Drug Information Center (APDIC).
Boesen says Arizona’s two poison centers document about 11,000 scorpion stings each year; 17,000 stings were reported to U.S. poison centers nationwide in 2009.
“We at the APDIC and University of Arizona College of Pharmacy are very excited (about Anascorp’s approval). I am proud of the expertise of the pharmacists and physicians working at the APDIC who helped make this research possible,” he says.
Anascorp was developed in Mexico and has been used there for many years, according to University of Arizona researchers who led the U.S. study of the drug. It’s made from the plasma of horses immunized with scorpion venom and vaccinated against viruses that could infect humans. Researchers began studying the drug in Arizona hospitals in 2004 and found it to be highly effective against the sting of the bark scorpion (also called the Arizona bark scorpion)—the most poisonous scorpion in the U.S.
Without Anascorp, children experiencing the most severe symptoms usually had to stay in intensive care in the hospital for several days; but when Anascorp was administered, researchers found patients’ symptoms disappeared after a few hours in the emergency room—eliminating the need for a hospital stay.
Bark Scorpion
The bark scorpion is found primarily in Arizona, but it also lives in other areas of the Southwest and northern Mexico, according to the Arizona-Sonora Desert Museum in Tucson, Ariz.
Scorpions are attracted to dark, moist spaces. They like to hide under rocks, wood, loose tree bark or anything else lying on the ground during the day, and they become active at night. Landscapers and others who work outside are at risk of being stung, as are people participating in outdoor activities.
Because they’re small and adept at climbing, scorpions may hitch a ride into homes in a sack of groceries or piece of clothing. Once indoors, they may get trapped in the sink or bathtub, look for a place to hide in an attic or crawl space, or scale the walls or ceiling, according to the desert museum website. Victims often report being stung while sleeping.
In June, a 6-month-old Arizona girl was airlifted from the small town of Oracle, Ariz., to University Medical Center in Tucson after a scorpion stung her as she slept, KSAZ-TV in Phoenix reported. Stephanie Moors, the child’s mother, was attending a yoga retreat and had just put her daughter down for a nap when she saw a scorpion’s tail wriggling under the child’s head. The girl was crying, vomiting, and, eventually, convulsing on the way to the hospital 36 miles away, but she made a full recovery after spending five days in the hospital.
The desert museum says you can check your home for scorpions by illuminating rooms with a black light flashlight or portable unit or a black light bulb in a lamp. Scorpion will glow a light blue-green color under the ultraviolet rays of a black light.
Jude McNally, the medical science liaison at Rare Disease Therapeutics, says the Tennessee company will market the new drug to any health care facility that accepts emergency patients in areas were the bark scorpion is found. That’s Arizona, as well as areas of Clark County, Nev., and parts of New Mexico where the bark scorpion has established colonies, he says.
Things to Know
The Arizona Poison and Drug Information Center says most stings to healthy, young adults can be managed at home with basic first aid and follow-up. Victims should
- clean the site with soap and water
- apply a cool compress
- elevate the affected limb to the same level as your heart
- take aspirin or acetaminophen as needed for minor discomfort
If a child is stung or the victim experiences severe symptoms, go to a medical facility immediately. If the child is under 5 years old or if an older patient is experiencing more than minor discomfort, call the poison center at 1-800-222-1222.
Made by Instituto Bioclon in Mexico City, Anascorp may cause early or delayed allergic reactions in people sensitive to horse proteins. The manufacturing process includes steps to decrease the chance of allergic reactions and to reduce the risk of transmission of viruses that may be present in the horse plasma.
FDA determined Anascorp was effective based on the results of an initial placebo-controlled, double-blind study of 15 children with neurological signs of scorpion stings. During placebo-controlled, double-blind studies, some patients get the medicine being tested, and others get a placebo—and even the researcher doesn’t know who gets which treatment. In the Anascorp study, symptoms disappeared within four hours in the eight subjects who received the antidote, but only one of the seven who received a placebo recovered so quickly.
In total, safety and efficacy data were collected from 1,534 patients in the studies led by the University of Arizona. The most common side effects of Anascorp were vomiting, fever, rash, nausea, itchiness, headache, runny nose, and muscle pain.
Experts say desert dwellers should know the symptoms of a scorpion sting and get treatment if severe symptoms develop. Severe symptoms include shortness of breath, fluid in the lungs, breathing problems, excess saliva, blurred vision, slurred speech, trouble swallowing, abnormal eye movements, muscle twitching, thrashing of the arms and legs, trouble walking, and other, uncoordinated muscle movements.
Source: U.S. FDA
03 January, 2012
Generic Drugs: Questions and Answers
What are generic drugs?
A generic drug is identical -- or bioequivalent -- to a brand name drug in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use. Although generic drugs are chemically identical to their branded counterparts, they are typically sold at substantial discounts from the branded price. According to the Congressional Budget Office, generic drugs save consumers an estimated $8 to $10 billion a year at retail pharmacies. Even more billions are saved when hospitals use generics.
Is there a generic equivalent for my brand-name drug?
To find out if there is a generic equivalent for your brand-name drug, use Drugs@FDA, a catalog of FDA-approved drug products, as well as drug labeling.
You can also search for generic equivalents by using the "Electronic Orange Book." Search by proprietary "brand" name," then search again by using the active ingredient name. If other manufacturers are listed besides the "brand name" manufacturer when searching by the "active ingredient," they are the generic product manufacturers.
Since there is a lag time after generic products are approved and they appear in the "Orange Book," you should also consult the most recent monthly approvals for "First Generics".
Are generic drugs as effective as brand-name drugs?
Yes. A generic drug is the same as a brand-name drug in dosage, safety, strength, quality, the way it works, the way it is taken and the way it should be used.
FDA requires generic drugs have the same high quality, strength, purity and stability as brand-name drugs.
Not every brand-name drug has a generic drug. When new drugs are first made they have drug patents. Most drug patents are protected for 20 years. The patent, which protects the company that made the drug first, doesn't allow anyone else to make and sell the drug. When the patent expires, other drug companies can start selling a generic version of the drug. But, first, they must test the drug and the FDA must approve it.
Creating a drug costs lots of money. Since generic drug makers do not develop a drug from scratch, the costs to bring the drug to market are less; therefore, generic drugs are usually less expensive than brand-name drugs. But, generic drug makers must show that their product performs in the same way as the brand-name drug.
How are generic drugs approved?
Drug companies must submit an abbreviated new drug application (ANDA) for approval to market a generic product. The Drug Price Competition and Patent Term Restoration Act of 1984, more commonly known as the Hatch-Waxman Act, made ANDAs possible by creating a compromise in the drug industry. Generic drug companies gained greater access to the market for prescription drugs, and innovator companies gained restoration of patent life of their products lost during FDA's approval process.
New drugs, like other new products, are developed under patent protection. The patent protects the investment in the drug's development by giving the company the sole right to sell the drug while the patent is in effect. When patents or other periods of exclusivity expire, manufacturers can apply to the FDA to sell generic versions.
The ANDA process does not require the drug sponsor to repeat costly animal and clinical research on ingredients or dosage forms already approved for safety and effectiveness. This applies to drugs first marketed after 1962.
What standards do generic drugs have to meet?
Health professionals and consumers can be assured that FDA approved generic drugs have met the same rigid standards as the innovator drug. To gain FDA approval, a generic drug must:
- contain the same active ingredients as the innovator drug(inactive ingredients may vary)
- be identical in strength, dosage form, and route of administration
- have the same use indications
- be bioequivalent
- meet the same batch requirements for identity, strength, purity, and quality
- be manufactured under the same strict standards of FDA's good manufacturing practice regulations required for innovator products
Source: FDA
Watching Life Develop From a Single Cell
Researchers at Yale University, in collaboration with the NIH and Sloan-Kettering recently developed a new, very powerful imaging technology that allows them to visualize with single cell resolution, the development of an organism in its entirety. This technology is 30 times faster than the fastest microscopes that are conventionally used in the field.
Source: NIHOD
How Do You Know If Your Child Has ADHD?

Is your child in constant motion? Does he or she talk incessantly? Or have trouble focusing and prefer to daydream?
Then your child may have attention deficit hyperactivity disorder, or ADHD.
This childhood disorder often begins between the ages of 3 and 6 years, according to the National Institute of Mental Health (NIMH). And it may continue through the teenage years and into adulthood.
Three types of ADHD are recognized:
- inattentive (trouble focusing, following instructions, and finishing tasks)
- hyperactive-impulsive (constantly on the go, talking excessively, and interrupting others)
- combined (symptoms of both inattention and hyperactivity-impulsivity)
Diagnosis
Studies show that the number of children being diagnosed with ADHD is increasing, and boys are more than twice as likely as girls to have it. According to the Centers for Disease Control and Prevention, as of 2007, about 9.5 percent of children 4 to 17 years old have at one time been diagnosed with ADHD.
Mark Ritter, M.D., R.Ph., who reviews drugs to treat ADHD at the Food and Drug Administration (FDA), explains the increase may be due to a greater public awareness of the disorder and psychiatric illnesses in general. And boys are more likely to have the hyperactive-impulsive type, which is easier to spot than the quieter child who is inattentive, says Ritter, who is also a practicing child psychiatrist.
Parents aren’t always aware of their child’s ADHD, says Ritter, until a teacher or other person outside the family suggests that the child may have it. “An educator may see that a child is fidgety, has problems focusing, and blurts out answers, and they have to spend an inordinate amount of time trying to keep the child still and focus the child’s attention.”
There is no single test to determine if a person has ADHD. A health care professional makes the diagnosis by comparing a person’s pattern of behavior against a set of criteria established by the American Psychiatric Association.
If you suspect your child might have ADHD, see your family doctor or pediatrician, suggests Ritter. Your child’s vision, hearing, and anything else that may contribute to inattention should be checked. The doctor may diagnose ADHD or refer your child to a mental health specialist for evaluation.
Treatments
FDA has approved two types of medications—stimulants and non-stimulants—to help reduce the symptoms of ADHD and improve functioning in children as young as 6 years.
“Stimulants have been the tried and true medication for ADHD since the 1950s,” says Ritter. “They have a long track record of being safe and effective.”
Despite their name, stimulants—which contain various forms of methylphenidate and amphetamine—actually have a calming effect on hyperactive children with ADHD, says NIMH. They are believed to increase levels in the brain of dopamine—a neurotransmitter associated with motivation, attention, and movement.
FDA has approved three non-stimulants to treat the symptoms of ADHD: Strattera (atomoxetine), Intuniv (guanfacine), and Kapvay (clonidine). Ritter says these provide a useful alternative for children. Some children do not tolerate stimulants well, and the non-stimulants do not have the same side effects.
Although stimulants are generally safe when taken as directed, FDA found 19 reported cases in a 5-year period of sudden death in children who took stimulants for ADHD. Roughly half of these children were also reported to have underlying structural heart defects, which raised a concern that the use of stimulants in such children might increase the risk of sudden death. In 2007, FDA required the drug label for all medications approved to treat ADHD—not just stimulants—to warn of this possible risk.
A large, recently completed study showed no evidence that ADHD drugs are associated with an increased risk of cardiovascular events (such as stroke, heart attack, and sudden cardiac death) in children and young adults. The medications studied include stimulants and the non-stimulants Cylert (pemoline), which is no longer sold, and Strattera. The study was sponsored by FDA and the Agency for Healthcare Research and Quality, another agency of the U.S. Department of Health and Human Services.
Medications do not cure ADHD, but control the symptoms. Some children outgrow ADHD, says Ritter, but medication is generally given through high school. No matter what drug is taken, it’s important to have the child regularly checked by the pediatrician, adds Ritter.
A study of nearly 600 children, ages 7 to 9, concluded that ADHD treatment with medication alone was more effective than behavioral therapy alone. The NIMH-sponsored study also found that behavioral therapy combined with medication was not more effective than medication alone to treat most ADHD symptoms.
However, the behavioral therapy was useful in helping to manage and modify some problem-causing behaviors. In addition, some children getting a combination of therapy and medication ended up taking lower doses of medication than those taking medication alone.
Consequences of Not Treating
Left untreated, ADHD can have serious consequences.
A child may fall behind in school, have difficulties that interfere with friendships, and have conflicts with parents, says the American Academy of Child and Adolescent Psychiatry.
Studies show that children with untreated ADHD have more emergency room visits and are more likely to have self-inflicted injuries than those treated for the disorder. Untreated adolescents with ADHD are more likely to take risks, such as drinking and driving. And they have twice as many motor vehicle accidents as those who are treated.
Source: FDA
LASIK Surgery and its Risks
The FDA developed this video to inform potential patients about the risks of LASIK. The video includes images of common visual problems that a LASIK patient may see.
Source: FDA
Fortify Your Knowledge About Vitamins (Consumer Update)
Although most people get all the vitamins they need from the foods they eat, millions of people take supplemental vitamins as part of their health regimen. This FDA Consumer Update video provides facts about vitamins, including information on how they are regulated. Learn more athttp://www.fda.gov/ForConsumers/ConsumerUpdates/ucm118079.htm
Making Antibodies That Neutralize HIV
Researchers have traced in detail how certain powerful HIV-neutralizing antibodies evolve, generating vital clues to guide the design of a preventive HIV vaccine.

Image: Illustration showing, in green, where the mature VRC01antibody binds to gp120 (red) on the surface of the HIV virus. Image by Wu et al., courtesy ofScience.
Most vaccines work by triggering the immune system to produce antibodies to help beat back infections. But proteins on the surface of HIV mutate rapidly, changing shape and preventing most antibodies from latching onto and neutralizing the virus.
Last year, scientists at NIH’s National Institute of Allergy and Infectious Diseases (NIAID) identified 2 HIV antibodies that could stop more than 90% of known global HIV strains from infecting human cells in the laboratory. Called VRC01 and VRC02, the antibodies were found in blood from an HIV-infected donor in North America. Led by NIAID’s Drs. Peter Kwong and John R. Mascola, the researchers subsequently discovered antibodies similar to VRC01 in the blood of 2 HIV-infected African donors.
The scientists found that the genes for VRC01-like antibodies undergo an unusually high number of mutations—70 to 90—between the first draft, which codes for a weak antibody, and the final version that codes for an antibody that can neutralize HIV. The scientists thus set out to map the route by which these antibodies develop. Their report appeared in the advanced online edition of Science on August 11, 2011.
Structural analysis revealed that the VRC01-like antibodies from the different donors all bind in the same way to the same spot on HIV. They attach to a molecule called gp120 in a region known as the CD4 binding site. HIV uses this site to attach to the cells it infects. It is one of the few parts of the virus that stays the same across HIV variants worldwide.
The genes for antibodies lie in the DNA of immune cells called B cells. To track the evolution of the antibody response to HIV at the genetic level, the researchers examined the B-cell DNA of 2 donors using a method called deep sequencing, along with sophisticated bioinformatics. Among hundreds of thousands of antibody genes, the researchers identified thousands that code for VRC01-like antibodies. They then sorted these genes into family trees showing their evolution from their earliest stage into mature forms. Next, the researchers focused on the gene segment that codes for the part of the antibody that attaches to and neutralizes HIV. They examined these sequences in detail to reveal how they changed step by step from their original state into a mature form.
“To make a vaccine that elicits VRC01-like antibodies, we will need to coach B cells to evolve their antibody genes along one of several pathways, which we have now identified, from infancy to a mature, HIV-fighting form,” says Dr. Gary J. Nabel, director of NIAID’s Vaccine Research Center.
Source: NIH
The scientists now aim to create proteins they can deliver through a vaccine to serve as signposts that direct the development of B-cell DNA to produce VRC01-like antibodies.
This research has far-reaching implications for vaccine development. “As we develop and test new HIV vaccines, it will be possible to analyze not just antibodies in the blood, but also the specific B-cell genes that are responsible for producing antibodies against HIV,” Mascola says. “This information will indicate whether an investigational HIV vaccine in a preclinical or clinical trial is heading in the right direction.”









